Trinuclear
1
Batholomew, A. K.; Teesdale, J. J.; Hernández Sánchez, R.; Malbrecht, B. J.; Juda, C. E.; Ménard , G.; Bu, W.; Iovan, D. A.; Mikhailine, A. A.; Zheng, S.-L.; Sarangi, R.; Wang, S. G.; Chen, Y.-S.; Betley, T.A. Proc. Natl. Acad. Sci., 2019, 116, 15836
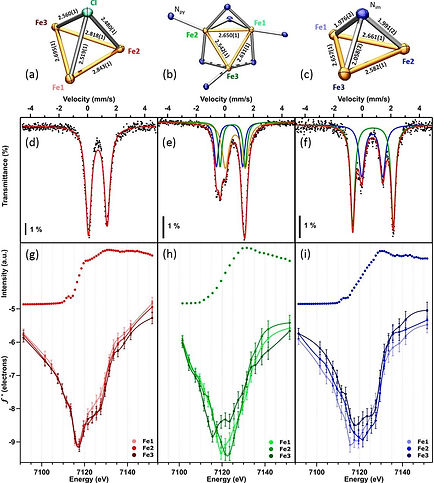
Synthesis. We have developed hexadentate, amine-based ligand scaffolds for the construction of trinuclear complexes. Using these ligands, we have synthesized trinuclear chromium, manganese, iron, cobalt, nickel, and copper complexes, featuring variable M-M interactions.
Electronic structure. Modifications of the ligand enable tuning of the electronic structure of these trinuclear complexes. Despite open-shell configurations, it is the direct M-M interactions, through orbital overlap, that dictate the changes in electronic structure. The tri-iron complexes in particular can achieve spin states of S = 1, 2, 4, 6, with spin-crossover allowing intermediate spin states to be accessed. These complexes has been isolated in eight different oxidation states, and characterized by proton H, Fe Mössbauer, SQUID magnetometry, and X-ray diffraction.
Cooperative redox and small molecule activation. The implementation of a ligand with bulky, peripheral -TBS groups
57
Trinuclear Complexes for Cooperative Redox Chemistry
Introduction. The development of polynuclear coordination complexes has given rise to structurally and electronically well-defined species possessing redox-flexibility with the potential to perform multi-electron transformations. The successful construction of these materials has allowed us to understand their redox behavior and target small molecule activation pathways reminiscent of naturally occurring enzymatic function.
restricts M-M bonding within the complexes, resulting in open-shell electronic configurations which render them reactive toward small molecules. Tri-iron complexes have been shown to reduce azides, hydrazine, and azobenzene under ambient conditions without the use of external reductants. Tri-manganese complexes in particular have been seen to extrude oxygen from O , N O and CO.
2 2

Tri-chromium clusters have recently been reported to perform varying site-isolated or cooperative redox chemistry depending on substrate sterics and solvents utilized in the reaction. We have also shown that the distribution of oxidation states within the trinuclear core can be examined using multiple-wavelength anomalous diffraction (MAD) upon conducting these redox transformations with these clusters.
Future Directions. Future work will focus on the preparation of reactive trinuclear complexes and determine how modifications of the ligand platform will impact both electronics and reactivity. Additionally, efforts have been made to synthesize the carbide adduct on our trinuclear platform. In an effort to understand the influence on reactivity that Lewis acids have in metalloenzyme active sites (OEC), mixed-metal trinuclear clusters incorporating both redox-inactive Lewis acidic metal ions (Zn, Ca) as well as redox-active metals (Fe, Co, Ni) are currently being investigated.
Bartholomew, A. K.; Juda, C. E.; Nessralla, J. N.; Lin, B.; Wang, S. G.; Chen, Y.; Betley, T. A. Angew. Chem. Int. Ed., 2019, 58, 5687